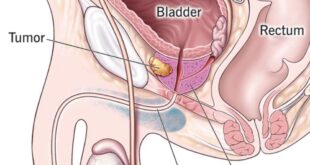
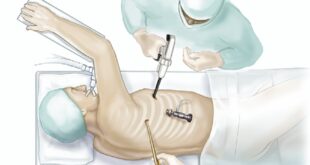
Prostate cancer is one of the most common cancers among men worldwide, and its management requires a comprehensive approach that goes beyond medical treatment. A …
Read More »Opdivo Treatment for Lung Cancer: A Complete Guide
Lung cancer remains one of the leading causes of cancer-related deaths worldwide, with millions of new cases diagnosed each year. For patients and families facing …
Read More »Adrenal Metastasis Lung Cancer: Understanding, Diagnosis, Treatment
Adrenal metastasis lung cancer is a condition where cancer cells from the lungs spread to the adrenal glands, located above the kidneys. This spread of …
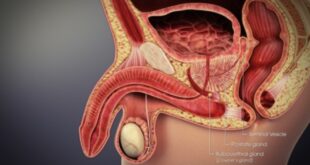
Read More »Prostate Cancer Extracapsular Extension Prognosis
Prostate cancer is one of the most common cancers affecting men worldwide, and its progression can vary greatly depending on the stage at diagnosis. Among …
Read More »Life Insurance After Breast Cancer: Causes and Risk Factors
Facing breast cancer is a life-changing journey that impacts not only physical health but also emotional and financial stability. For many survivors, one of the …
Read More »Breast Shrinking Cancer: Symptoms, Diagnosis, Treatment
Breast health is a critical concern for women worldwide, and changes in the size, shape, or texture of breast tissue often raise important medical questions. …
Read More »Tarceva Pancreatic Cancer: A Comprehensive Guide
Pancreatic cancer is one of the most aggressive and challenging cancers to treat, often diagnosed at an advanced stage. In recent years, targeted therapies have …
Read More »Metastatic Prostate Cancer Treatment: A Complete Guide
Prostate cancer is one of the most common cancers affecting men worldwide, and when it progresses to an advanced stage, it can spread beyond the …
Read More »Treatment for Lung Cancer in the Elderly: Causes and Risk Factors
Lung cancer remains one of the most common and serious health conditions affecting older adults worldwide. As life expectancy increases, more elderly patients are being …
Read More »