Stagescancer.net – The landscape of leukemia treatment is witnessing a significant shift with the introduction of FLT3 inhibitor therapy, a groundbreaking development in the fight against hematological malignancies. Through targeted approaches that challenge traditional methodologies, FLT3 inhibitors are carving a niche in precision medicine, offering hope to patients with specific genetic mutations associated with aggressive forms of cancer. This article explores the advancements in cancer therapy that FLT3 inhibitors represent, promising improved survival rates and outcomes for patients contending with these relentless diseases.
The Role of FLT3 in Cancer Biology
Understanding the role of oncogenes in cancer biology is essential for the advancement of targeted treatments. One such oncogene, FLT3 (Fms-like tyrosine kinase 3), is a receptor tyrosine kinase that is instrumental in the development of hematopoietic stem cells and immune cell lineages. In its normal stage-4-cancer-life-expectancy-without-treatment/” title=”Baca lebih lanjut tentang state”>state, FLT3 facilitates the growth and maturation of these essential cells. However, when FLT3 mutations occur, this ordinarily beneficial gene can abet the onset and cancer growth, particularly in hematological malignancies such as acute myeloid leukemia (AML).
Several types of mutations in the FLT3 gene have been identified, with internal tandem duplications (FLT3-ITD) being the most common in AML. These mutations lead to constitutive activation of the FLT3 protein, which in turn signals a cascade of events promoting the survival, proliferation, and differentiation blockage of leukemic cells. As a result, FLT3 mutations are associated with a poorer prognosis due to a higher likelihood of disease relapse.
Given the pivotal role that FLT3 plays in the pathogenesis of certain cancers, it has emerged as one of the key molecular targets in oncology. The discovery of FLT3’s influence on cancer pathology has paved the way for a new class of drugs specifically designed to inhibit the aberrant activity caused by FLT3 mutations. These targeted therapies aim to derail the mechanism that enables leukemic cells to thrive, thus restoring the regulation of cell growth and leading to cancer regression.
- Targeting FLT3 has become a focal point of precision medicine, which seeks to provide more individualized and effective treatment plans.
- FLT3 inhibitors show promise in delivering impactful therapeutic interventions and improving clinical outcomes in patients with FLT3-driven cancers.
- The ongoing research into FLT3 and its inhibitors continues to provide valuable insights into overcoming resistance to therapy, enhancing drug efficacy, and ultimately, improving the survival rates of patients affected by hematologic cancers.
As oncology continues to evolve toward more precise molecular targeting, the importance of understanding FLT3’s role within cancer biology not only enables the development of new therapeutic strategies but also exemplifies the broader trend of honing in on genetic abnormalities that can be leveraged for the advancement of personalized medicine.
Understanding FLT3 Inhibitors
In the realm of oncology, FLT3 inhibitor drugs are paving the way for targeted cancer treatments, offering hope to patients with specific genetic profiles. These kinase inhibitors are central to the pursuit of precision medicine, representing a shift away from general chemotherapy to treatments tailored to individual genetic anomalies.
What Are FLT3 Inhibitors?
FLT3 inhibitors are innovative pharmacological agents engineered to thwart the FLT3 enzyme, a type of receptor tyrosine kinase whose mutation is implicated in the proliferation of various blood cancers. By impeding the FLT3 pathway, these drugs disrupt the cascade of signals essential for the survival and unchecked division of cancer cells possessing FLT3 mutations – an intricate process that underscores their value in the arsenal against cancer.
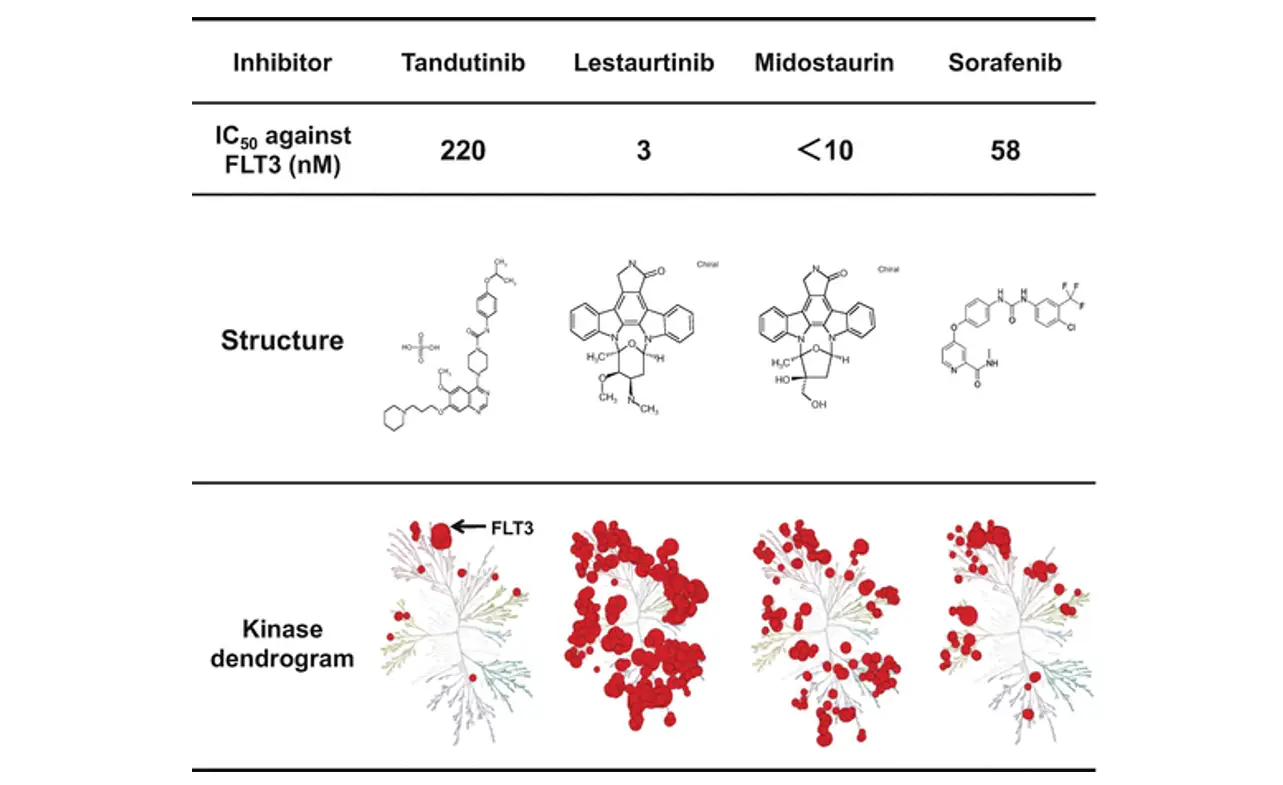
Types of FLT3 Inhibitors
The landscape of FLT3 inhibitors is marked by diversity, with drugs categorized by their mechanism of action and their specificity. Understanding their classification can help physicians tailor targeted cancer treatments more effectively:
- Selective FLT3 inhibitors are geared specifically to target the FLT3 protein alone.
- Multitargeted inhibitors cast a wider net, affecting FLT3 among other kinases.
Their classification extends to their binding affinity:
- Type I FLT3 inhibitors connect with the active conformation of the FLT3 receptor, effectively inhibiting its function when the receptor is in an active state.
- Type II FLT3 inhibitors block the receptor in an inactive conformation, preventing it from becoming activated and igniting the downstream signaling that fuels cancer cell survival and multiplication.
FLT3 Inhibitor: The Breakthrough in Targeted Cancer Therapy
The era of precision oncology has been markedly advanced by the introduction of FLT3 inhibitors, a development that has reshaped the landscape of breakthrough cancer treatment. As strategic agents in the fight against acute myeloid leukemia (AML), these targeted therapeutic options have brought about a pivotal transition from classical chemotherapy to treatments that are more finely tuned to individual genetic profiles. This specialization in care stems from an intimate understanding of the genetic mutations that propel cancer progression, placing FLT3 inhibitors at the forefront of advanced leukemia therapy.
Contributing to this innovative leap in medicine, these inhibitors have facilitated a significant upsurge in remission rates among patients with FLT3-mutated AML. The targeted approach of FLT3 inhibition effectively hinders the growth and sustenance of cancer cells by impeding the specific mutations that drive the disease. This has not only improved response rates but has also laid the groundwork for next-stage combination therapies—tactics that promise to amplify the impact of treatment and better manage resistance.
- Increased Precision: Catering treatments to individual genetic aberrations.
- Enhanced Efficacy: Offering an improved alternative to conventional treatments.
- Combination Opportunities: Laying the groundwork for synergistic treatment approaches.
- Resistance Management: Opening avenues to tackle treatment resistance.
In summary, FLT3 inhibitors represent more than just an advancement in medical treatment; they serve as a beacon of hope for a future dominated by patient-customized therapies that not only treat but also aspire to outpace the complex nature of cancer.
| Therapeutic Aspects | Benefits of FLT3 Inhibitor Therapy | Impact on AML Treatment Paradigm |
|---|---|---|
| Targeted Action | High precision in attacking FLT3-mutated cells | Shift from broad-spectrum chemotherapy to focused therapy |
| Combination Regimens | Potentiation of efficacy; reduction of resistance | Enhanced remission rates; potential for longer-term management of AML |
| Patient Response | Increased remission rates and improved survival | Personalized medicine becomes a reality for AML patients |
Mechanisms of Action: How FLT3 Inhibitors Work
The development of FLT3 inhibitors has revolutionized the approach to treating hematological malignancies, specifically acute myeloid leukemia (AML). These inhibitors function through two primary pathways: blockade of the FLT3 signaling and induction of apoptosis in AML cells. This dual mechanism profoundly impacts the survival of cancer cells, transforming treatment protocols and patient outcomes.
Inhibiting the FLT3 Signaling Pathway
FLT3 inhibitors are designed to zero in on the ATP-binding site found within the FLT3 kinase domain, a critical juncture in the FLT3 pathway frequently associated with AML. By binding to this site, the inhibitors forestall the phosphorylation and activation of FLT3, thereby disrupting the downstream signaling cascade essential for the multiplication and persistence of cancer cells. The targeted molecular therapy approach underscores the specificity of FLT3 inhibitors as they hone in on aberrant pathways, markedly improving the treatment landscape for patients with AML.
Inducing Apoptosis in Cancer Cells
Another significant action of FLT3 inhibitors lies in their ability to trigger apoptosis—programmed cell death—which is a pivotal process in targeting and eliminating malignant cells. FLT3 inhibitors enhance the activity of pro-apoptotic pathways while simultaneously repressing anti-apoptotic proteins. This strategic approach tips the scales toward cellular death, thereby reducing tumor burden. With targeted molecular therapy, FLT3 inhibitors apply a one-two punch in treating AML, not only halting cell proliferation but also actively promoting the demise of cancer cells.
| FLT3 Inhibitor Function | Targeted Pathway | Therapeutic Impact |
|---|---|---|
| Inhibition of FLT3 Kinase Activation | FLT3 Signaling Pathway | Hinders cancer cell proliferation and survival |
| Induction of Apoptosis | Pro-apoptotic signaling pathways | Accelerates programmed cancer cell death |
| Suppression of Anti-apoptotic Proteins | Cellular survival pathways | Disrupts cancer cell defense mechanisms |
Through the systematic blocking of the FLT3 pathway and promoting apoptosis in AML, FLT3 inhibitors have emerged as a cornerstone of targeted molecular therapy in the fight against leukemia. Their application represents a crucial advancement in precision oncology, offering hope for higher remission rates and the potential for long-term disease control.
The Clinical Efficacy of FLT3 Inhibitors in Leukemia
The landscape of acute myeloid leukemia (AML) treatment has been profoundly impacted by the advent of FLT3 inhibitors, which have been instrumental in improving clinical outcomes for patients with FLT3-positive AML. Evidence accrued from rigorous clinical trials has illustrated the substantial increase in therapeutic efficacy these targeted drugs offer, especially when measured against conventional chemotherapy protocols.
Advancements made with FLT3 inhibitors have redefined treatment expectations, showing not just incremental, but significant strides in complete remission rates. This boon to patient care is further underlined by the amplification of overall survival spans seen in various studies, which positions FLT3 inhibitors as a cornerstone of modern leukemia therapy.
- Higher complete remission rates among patients
- Prolongation of overall survival
- Validation of FLT3 as a viable therapeutic target
Continuous research endeavors strive to pinpoint the most effective dosing, considering the heterogenic nature of FLT3 mutations in the patient population. This relentless pursuit aims to optimize the long-term utilization of FLT3 inhibitor therapy, ensuring these medical advances translate into tangible survival benefits for AML patients.
Development of FLT3 Inhibitor Drugs
The journey from conceptualizing FLT3 inhibitors to ushering them into the clinic is a testament to the innovation in drug discovery, with pivotal advances manifesting through rigorous laboratory research and subsequent FLT3 inhibitor development. A steadfast commitment to addressing the complexities of FLT3 gene mutations has propelled these medications toward clinical trial advancements, promising a new horizon in targeted leukemia treatment.
From Laboratory to Clinical Trials
Embarking on the path from laboratory discovery to clinical application, FLT3 inhibitors have undergone extensive preclinical research. Scientists strive to isolate compounds with potent inhibitory effects specifically targeted against FLT3 mutations. This meticulous process of identification sets the stage for the structured evolution of these potential therapeutics through the phases of clinical trials, systematically analyzing safety profiles, efficacious dosing, and the significant impact on affected patient populations.
Challenges in Drug Development
Navigating drug development is fraught with challenges, particularly for FLT3 inhibitors, where the goal is to fine-tune selectivity to mitigate off-target effects. Additionally, the inherent complexity of drug resistance presents a formidable hurdle, necessitating creative approaches to drug combinations and dosing strategies. Addressing the diversity of FLT3 mutations means individualized treatment constructs must be developed, ensuring the heightened efficacy of these targeted therapies. A thorough understanding of the pharmacokinetic and pharmacodynamic attributes of these drugs is essential for guaranteeing a maximized therapeutic advantage to patients in need.
Combination Therapies: Enhancing the Effectiveness of FLT3 Inhibitors
The emerging landscape in optimized leukemia therapy is witnessing an exciting shift towards the use of combination treatment strategies. By integrating FLT3 inhibitors with additional cancer-fighting agents such as traditional chemotherapy, newer targeted therapies, and innovative immunotherapeutics, oncologists are discovering the power of synergistic effects. These combinations are paving the path forward in offering patients a comprehensive, multi-angle assault on cancer cells.
The rationale behind these combination therapies is not solely to combine forces but to meticulously craft regimens that can minimize the likelihood of drug resistance—a notorious challenge in leukemia treatment—and subsequently heighten overall response rates. With evidence mounting through clinical trials, the goal is to identify the most promising combinations that unlock an optimized leukemia therapy approach for those affected by FLT3-mutant AML.
| Combination Approach | Potential Benefits | Current Research Focus |
|---|---|---|
| FLT3 Inhibitors + Chemotherapy | Enhanced efficacy versus chemotherapy alone | Assessing optimal dosing and timing schedules |
| FLT3 Inhibitors + Targeted Drugs | Reduced toxicity, targeted attack on cancer cells | Identifying effective drug pairings for specific FLT3 mutations |
| FLT3 Inhibitors + Immunotherapy | Stimulation of immune response against cancer cells | Evaluating long-term outcomes and immune-related side effects |
As we advance, it becomes clear that FLT3 inhibitor therapy is not a stand-alone option but part of a broader, dynamic puzzle. The integration of these drugs into well-designed combination regimens holds the promise of delivering enhanced care to patients, tapping into the potential for truly optimized leukemia therapy.
Overcoming Resistance to FLT3 Inhibitor Treatment
As FLT3 inhibitors have become a cornerstone in the management of hematological malignancies, the phenomenon of treatment resistance presents a significant hurdle. Addressing this challenge is critical for enhancing patient outcomes and achieving a durable response in therapy. Understanding the factors that contribute to overcoming drug resistance is paramount in the development of effective treatment strategies.
Mechanisms of Resistance
Several mechanisms underlie resistance to FLT3 inhibitor treatment. Researchers have discovered that secondary mutations within the FLT3 gene can alter the binding site, rendering the inhibitor less effective. Moreover, cancer cells may activate alternative growth and survival signaling pathways or experience pharmacokinetic issues that result in inadequate drug concentrations at the cellular level. These complications are key contributors to the reduced efficacy of FLT3 inhibitors over time and can expedite disease progression if not properly managed.
Strategies to Counteract Resistance
In pursuit of overcoming drug resistance, efforts to establish more resilient treatment options have intensified. Among these, the design of second-generation FLT3 inhibitors that provide broader activity against a variety of mutations is progressing. Moreover, the precise optimization of dosing regimens is seen as a vital piece in sustaining drug effectiveness. The synergy obtained through combination therapies also offers promise, potentially averting the onset of resistance.
To complement these approaches, a proactive stance on monitoring for resistance mutations has been adopted to inform therapeutic adjustments promptly. The tabulated representation below illustrates the major strategies that are being explored to achieve lasting responses to FLT3 inhibitor therapy:
| Strategy | Purpose | Benefits |
|---|---|---|
| Second-Generation Inhibitors | Target a broader range of FLT3 mutations | Improved efficacy against diverse mutations |
| Optimized Dosing Regimens | Maintain optimal drug concentrations | Enhanced drug activity and reduced toxicity |
| Combination Therapies | Attack multiple pathways simultaneously | Synergistic effects; reduced resistance risk |
| Preemptive Monitoring | Early detection of resistance mutations | Immediate therapeutic intervention to circumvent resistance |
In summary, achieving a durable response in therapy requires an integrated approach that incorporates the latest scientific insights into resistance mechanisms. By utilizing targeted strategies and individualized patient care, the goal of effectively overcoming drug resistance becomes an attainable objective in the treatment of hematological malignancies.
Side Effects and Management in FLT3 Inhibitor Therapy
The introduction of FLT3 inhibitors into leukemia treatment protocols has represented a significant stride forward in precision medicine, yet it also necessitates astute toxicity management. Patients undergoing this innovative cancer therapy may encounter a range of treatment side effects that can significantly impact their quality of life. Commonly reported adverse effects include, but are not limited to, gastrointestinal disturbances, blood cell count abnormalities, skin reactions, and hepatic enzyme elevations.
To ensure optimal patient care, a balance must be struck between the therapeutic benefits of FLT3 inhibitors and the proactive mitigation of adverse reactions. Management strategies tailored to the severity of the side effects include comprehensive supportive care measures, judicious dose modifications, and, in certain scenarios, temporary or permanent discontinuation of the therapy. Educating patients on the potential side effects and the importance of rapid reporting is also critical to the successful management of these inhibitors.
The table below outlines a structured approach to managing common side effects associated with FLT3 inhibitor therapy:
| Side Effect | Management Strategy | Patient Counseling Points |
|---|---|---|
| Gastrointestinal Symptoms | Medication for nausea, diet modification, hydration | Report persistent symptoms, the importance of staying hydrated |
| Cytopenias | Monitoring blood counts, dose adjustment, growth factor support | Understand infection risks, and report signs of anemia or bleeding |
| Rash/Skin Reactions | Topical treatments, antihistamines, dose modification | Monitor skin condition, avoid direct sunlight |
| Elevated Liver Enzymes | Regular liver function tests, dose adjustment, hepatoprotective agents | Report yellowing of the skin or eyes, unusual fatigue |
Care providers must remain vigilant in toxicity management, continually adjusting therapeutic regimens in response to evolving patient needs. This dynamic approach aims to sustain the promised benefits of FLT3 inhibitors, ensuring a marked improvement in the quality of life for patients battling leukemia.
Survival Rates and Prognosis with FLT3 Inhibitor Use
The introduction of FLT3 inhibitors has marked a significant stride forward in the treatment landscape of acute myeloid leukemia (AML). Numerous studies have shed light on the prognostic implications of FLT3 mutations and the therapeutic impact of targeting these mutations. Notably, the adoption of FLT3 inhibitors has heralded an era where measured improvements in long-term outcomes are observable, shifting the dynamics of survivorship in this patient demographic.
As an important prognostic factor, the presence of a FLT3 mutation in AML cases has traditionally been associated with a more aggressive disease course and a reduced likelihood of survival. However, with the advent of FLT3 inhibitor therapy, the narrative is taking a turn. The emergence of these agents has offered hope through the observed FLT3 inhibitor survival benefit, particularly when these inhibitors are used in combination treatments, creating a synergy that propels their effectiveness.
- Enhanced remission rates through combination therapy
- Extended disease-free intervals post-treatment
- Improved overall survival statistics
Such advancements have inherently laid the groundwork for more nuanced therapeutic decisions, with FLT3 mutation status now serving as a pivotal compass in the direction of AML management strategies. Continued assessment and longitudinal studies are essential in evaluating the full scope of these inhibitors’ benefits, delving into the depths of survivability figures, and exploring the bounds of achievable quality-of-life improvements for those affected.
It is imperative, therefore, that the medical community persists in its scrutiny and analysis of FLT3 inhibitor performance. The imperative journey from clinical trials to real-world applications offers a promising vista of accruing data. This data not only enhances our collective understanding of these agents’ efficacy but more crucially, informs our shared objective of elevating patient care standards, reinforcing the goal of achieving sustained remissions and, ultimately, cures in hematologic oncology.
Current Research and Future Directions in FLT3 Inhibition
The landscape of cancer treatment is being reshaped by cutting-edge research focused on the use of FLT3 inhibitors, addressing fundamental challenges and unraveling new possibilities in combating hematological malignancies. The quest for an expansion in the therapeutic reach of FLT3 inhibitors is diligently pursued within the confines of intricate clinical trials. These ventures are pivotal to accruing clinical trial insights that have the potential to augment the efficacy and application of FLT3 inhibitors.
Ongoing Clinical Trials
Spearheading this innovative frontier, continuous clinical studies are instrumental in probing the capabilities of FLT3 inhibitors. New compounds and combination treatments undergo rigorous evaluations to establish protocols that aim to prevent or surmount resistance to therapy. Incremental advancements from each study refine the practice of targeted treatments, aiming to amplify prognosis for patients with FLT3-mutated AML.
The Potential of FLT3 Inhibitors in Other Cancers
The horizon for FLT3 inhibitor expansion extends its reach beyond the borders of AML. Intensive research elucidates their efficacy against diverse hematological malignancies which demonstrate FLT3 involvement, and intriguingly, their role in challenging solid tumors. The burgeoning potential foreseen from these studies hints at a broader spectrum of indications for FLT3 inhibitors, underscoring their versatility as a pivotal tool in oncological therapeutics.
Regulatory Approvals and Market Availability of FLT3 Inhibitors
The journey towards FDA approval has been a cornerstone in facilitating global market access for FLT3 inhibitors, a novel class of drugs transforming the treatment of FLT3-mutated AML. As part of the intricate medical regulatory landscape, various agencies vigilantly evaluate clinical data to ensure the efficacy and safety of these groundbreaking cancer therapies.
Amidst regulatory achievements, FLT3 inhibitors have garnered approvals across continents, offering a beacon of hope to many battling hematological malignancies. Following the FDA’s nod, other international regulatory bodies have rigorously assessed these new drugs, recognizing the impact of FLT3 inhibitor treatments on patient outcomes.
Here is an overview of the current state of approvals and availability for FLT3 inhibitors in key markets:
| Region | Regulatory Authority | Status of Approval | Remarks |
|---|---|---|---|
| United States | Food and Drug Administration (FDA) | Approved | For the treatment of FLT3-mutated AML. |
| European Union | European Medicines Agency (EMA) | Approved | Inclusion in treatment protocols for AML. |
| Japan | Pharmaceuticals and Medical Devices Agency (PMDA) | Approved | Utilized in specific AML patient subsets. |
| Canada | Health Canada | Approved | Accessible to the Canadian patient population. |
| Australia | Therapeutic Goods Administration (TGA) | Under Review | Pending approval based on clinical trial outcomes. |
As FLT3 inhibitors gain traction, continuous scrutiny and updates from regulators are expected. These concerted efforts aim to optimize treatment protocols, ultimately enhancing the quality of care for patients worldwide.
Patient Selection and Criteria for FLT3 Inhibitor Therapy
In the realm of precision medicine, the selection of patients for FLT3 inhibitor therapy represents a cornerstone of effective treatment for hematological malignancies. Establishing the criteria that dictate patient eligibility is driven by a multifaceted assessment process geared toward enhancing therapeutic decision-making. This process is intricate, ensuring that only those most likely to benefit are chosen for this targeted approach. Ensuring patients meet the necessary criteria allows for a more tailored treatment likely to result in better outcomes and fewer side effects.
- Assessment of FLT3 Mutation Status: A critical determinant in therapy eligibility, requiring comprehensive genetic profiling to identify candidates.
- Disease Characteristics: Detailed examination of leukemia subtype, mutation burden, and stage of the disease.
- Treatment History: Evaluation of prior treatments and responses to gauge potential benefits from FLT3 inhibitors.
- Overall Health Assessment: General health and fitness levels to endure treatment are considered imperative for eligibility.
To distill these selection parameters, a multidisciplinary team collaborates to align treatment plans with the unique needs of each patient. This collective expertise forms the bedrock of personalized care that precision medicine advocates for, ensuring therapies are both targeted and effective. Hence, FLT3 inhibitor therapy becomes not just a possible intervention but a judicious decision shaped by a thorough and robust criterion.
FLT3 Inhibitor: A Paradigm Shift in Hematology Oncology
The inception of FLT3 inhibitor therapy marks a significant milestone in the transformative cancer therapy landscape. Known to be at the forefront of oncology treatment evolution, FLT3 inhibitors have introduced a targeted approach to combat blood-related malignancies with noteworthy precision. As the adoption of these next-generation therapies gains momentum, it is undeniable that the era of one-size-fits-all treatment is being eclipsed by a new dawn of individualized care.
Historically, hematology-oncology faced the challenge of treating diverse patient populations with a limited arsenal of broad-spectrum chemotherapies. This often resulted in variable efficacy and significant off-target effects. However, ramping up our understanding of cancer’s molecular underpinnings has taken us down an optimistic path where targeted therapies like FLT3 inhibitors are changing the game, bringing us closer to the ideal of precision medicine.
Here is a snapshot of how FLT3 inhibitors are reshaping the therapeutic landscape:
- Enabling Precision: By honing in on specific genetic mutations, these therapies offer a customized treatment regime.
- Improving Outcomes: There is a marked improvement in patient response rates and overall survival, indicating a shift in the trajectory of disease management.
- Setting New Standards: The integration of FLT3 inhibitors paves the way for their inclusion in standard care protocols, presenting an advanced blueprint for targeted cancer care.
Future horizons in cancer treatment are expanding as ongoing research perpetuates the design and development of even more sophisticated FLT3 inhibitor-based interventions. One can foresee a continuing evolution where predictive diagnostics, innovative drug formulations, and personalized treatment regimens domicile themselves as foundational elements in the fight against cancer.
Undoubtedly, FLT3 inhibitor therapy stands as a testament to the relentless pursuit in oncology to diminish the burdens of disease and magnify the hope for a cure. As we navigate through this transformative age in cancer therapy, it is incumbent on all healthcare providers to stay abreast of these pivotal advances and incorporate them into clinical practice for the betterment of patient care across the globe.
Key Takeaways for Healthcare Professionals
As the spectrum of cancer therapies broadens with the introduction of FLT3 inhibitors, healthcare providers are entrusted with a pivotal role in the interpretation and application of these treatments within oncology practice. The incorporation of FLT3 inhibitors into patient care necessitates a refined understanding of their clinical implications, effective application in precision medicine, and vigilant management of any associated adverse effects. A thorough comprehension of the molecular underpinnings of FLT3-related hematologic malignancies is essential for maximizing the therapeutic potential of these agents.
Within the dynamic landscape of FLT3 inhibitor therapy, the significance of identifying FLT3 mutation status as an integral element of diagnostic evaluation for acute myeloid leukemia (AML) cannot be overstated. With FLT3 mutations playing a critical role in disease progression and prognosis, timely and accurate assessment is crucial in shaping treatment regimens. Moreover, healthcare professionals should endeavor to keep abreast of the evolving FLT3 inhibitors guidance to furnish their patients with advanced leukemia treatment options that are tailored to their unique genetic profiles.
In the realm of oncology, a collaborative, interdisciplinary approach coupled with ongoing education forms the cornerstone of successful patient outcomes. The concerted efforts of specialists are central to the seamless integration of FLT3 inhibitors into comprehensive cancer care protocols. As the therapeutic landscape transforms with these advancements, maintaining a patient-centered framework is paramount. By adopting these practices, healthcare providers ensure that the promises held by FLT3 inhibitor therapies are fully realized, thus spearheading progress in the fight against hematological malignancies.
FAQ
What are FLT3 inhibitors and how do they work in treating hematological malignancies?
FLT3 inhibitors are a class of targeted therapy drugs that impede the FLT3 gene’s enzyme activity, which is often mutated in hematological cancers like acute myeloid leukemia (AML). By blocking this activity, these therapies inhibit cancer cell proliferation and increase survival rates by preventing disease progression and relapse.
How has the discovery of FLT3 mutations impacted cancer biology?
The identification of FLT3 mutations has underscored the gene’s role as a critical oncogene in cancers like AML, where it enhances cell survival and proliferation. This has transformed the approach to precision medicine in oncology, allowing for the development of targeted therapies aimed specifically at mutated FLT3 and potentially improving treatment efficacy.
Can you elaborate on the types of FLT3 inhibitors and their mechanisms?
FLT3 inhibitors can be selective, targeting only the FLT3 enzyme, or multitargeted, impacting various kinases. They can also be categorized based on their interaction with the FLT3 protein—type I inhibitors bind to the active conformation, while type II targets the inactive conformation, preventing activation.
What constitutes the breakthrough in targeted cancer therapy with FLT3 inhibitors?
FLT3 inhibitors have marked a significant advancement in cancer treatment, emphasizing the principles of precision oncology. By targeting specific genetic mutations, they offer an alternative to traditional chemotherapies and can be used in combination treatments to enhance their efficacy and surmount drug resistance.
How does the inhibition of the FLT3 signaling pathway contribute to treating leukemia?
Inhibiting the FLT3 signaling pathway disrupts the signals essential for the survival and growth of cancer cells. FLT3 inhibitors prevent phosphorylation and activation of downstream molecules, ultimately leading to the suppression of tumor progression and, in some cases, inducing programmed cell death or apoptosis in cancer cells.
What evidence supports the clinical efficacy of FLT3 inhibitors in treating leukemia?
Clinical trials have demonstrated that FLT3 inhibitors significantly improve outcomes for patients with FLT3-mutated AML, offering higher remission rates and enhanced overall survival compared to conventional chemotherapy. Their success has led to regulatory approvals and their inclusion in clinical guidelines for AML treatment.
What challenges do researchers face in the development of FLT3 inhibitor drugs?
Challenges include achieving selective targeting to minimize off-target effects, mitigating drug resistance, and optimizing drug combinations. Additionally, researchers must consider the molecular heterogeneity of FLT3 mutations and ensure favorable pharmacokinetic and pharmacodynamic profiles for these agents.
How can combination therapies improve the effectiveness of FLT3 inhibitors?
Combination therapies can lead to synergistic effects by targeting cancer from multiple angles, reducing resistance, and amplifying response rates. Research into these combinations aims to find the most effective regimens to enhance outcomes for patients with FLT3-mutant AML.
What are the strategies to overcome resistance to FLT3 inhibitor treatment?
Strategies include developing second-generation inhibitors with wider activity, refining dosing regimens, applying combination therapies, and monitoring for resistance mutations. A nuanced understanding of resistance mechanisms is key to achieving lasting and effective responses to FLT3 inhibitor therapy.
What are some common side effects of FLT3 inhibitor therapy and how are they managed?
Side effects can range from gastrointestinal symptoms to cytopenias and elevated liver enzymes. Managing these involves supportive care, adjusting dosages, or potentially halting the drug, emphasizing the need for proactive monitoring and patient education about potential adverse events.
What does the use of FLT3 inhibitors mean for the survival rates and prognosis of AML patients?
FLT3 inhibitors are associated with improved survival rates and prognostic outcomes, serving as a vital biomarker that guides therapeutic decisions. Long-term studies are vital to understanding their full impact on survival and disease-free periods.
What is the current status of clinical research on FLT3 inhibitors?
Current research is focused on assessing new compounds, combination strategies, and preventive measures for drug resistance through ongoing clinical trials to further optimize FLT3 inhibitor use in leukemia and explore their utility in other malignancies.
How has the regulatory approval of FLT3 inhibitors affected their market availability?
FLT3 inhibitors have gained regulatory approvals worldwide, including FDA approval for FLT3-mutated AML treatment in the US. This has introduced a significant option in the oncology market, although global access and healthcare disparities are considerations for expanding the benefits of these treatments.
What criteria determine patient eligibility for FLT3 inhibitor therapy?
Crucial factors include the type and burden of FLT3 mutations, previous treatments, and the likelihood of benefiting from targeted therapy, necessitating comprehensive genetic profiling and a multidisciplinary approach in therapeutic decision-making.
How do FLT3 inhibitors signal a paradigm shift in hematology-oncology?
FLT3 inhibitors exemplify the shift towards targeted treatment approaches within hematology-oncology, reflecting an era of precision medicine with the potential to significantly improve patient outcomes through molecular targeting.
What key takeaways should healthcare professionals bear in mind regarding FLT3 inhibitors?
Healthcare professionals should be versed in the appropriate use of FLT3 inhibitors, side effect management, and resistance mechanisms. They should also incorporate FLT3 mutation status in diagnostic and treatment protocols, ensuring ongoing education and patient-centered care for the best outcomes.